Frequently Asked Questions
Is any contaminating genomic DNA present in the RNA obtained using the RNAstorm™ kit?
Contamination from genomic DNA is a big concern because it can intefere with downstream applications. The RNAstorm™ kit includes an optimized DNase digestion step which removes contaminating genomic DNA without significantly affecting RNA yield. While this step is optional, it is highly recommended.
How much RNA can I expect to obtain from an FFPE sample?
The biggest variable that affects the total amount of RNA obtained is the quality of the sample itself (i.e. the type and amount of tissue, and the care taken in isolation and preservation of the sample). Using the RNAstorm™ kit, and assuming at least reasonable sample quality, amounts greater than 1 µg can be obtained.
Can RNA obtained using the RNAstorm™ kit be used in RNA-Seq?
Yes. Good quality libraries can be obtained, providing that the RNA is of sufficiently high quality. For Illumina sequencing, a DV200 of at least 30% is recommended, and samples should be used that provide at least 1 µg of RNA.
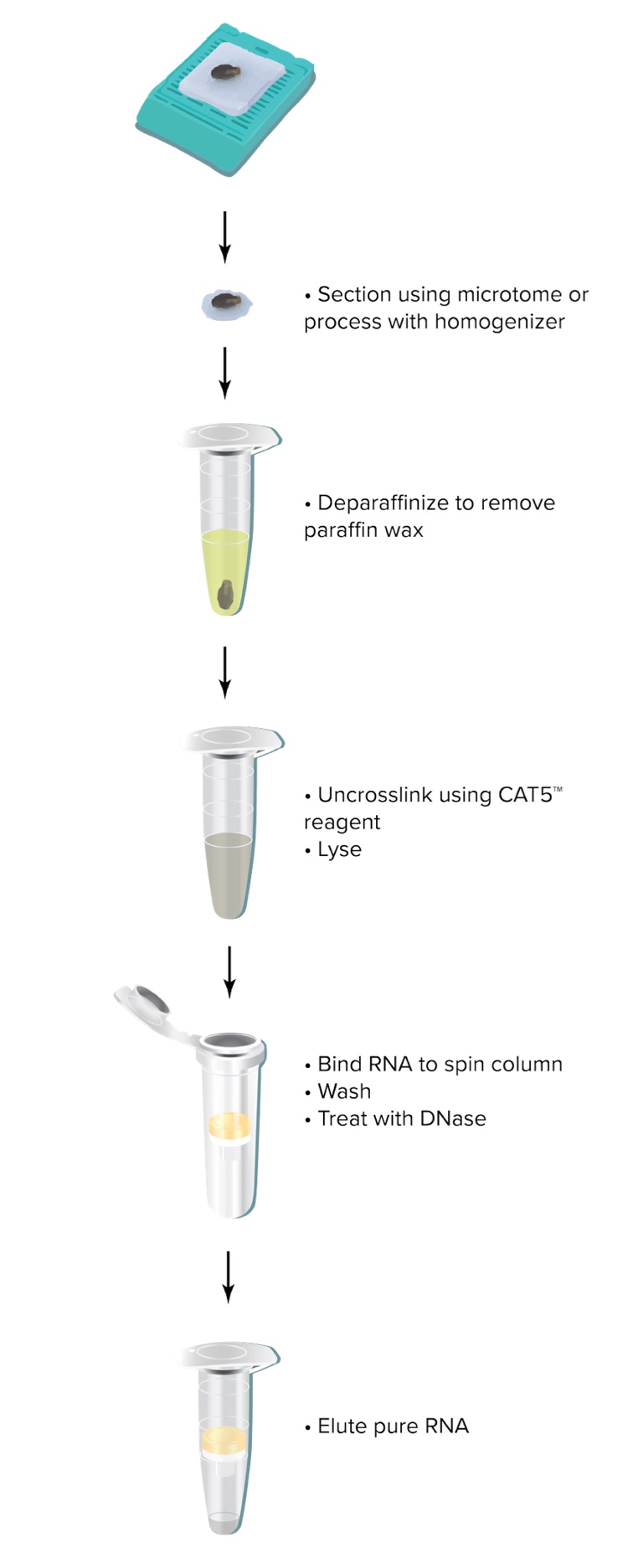
How should the tissue be prepared?
Use a microtome to obtain 5-10 µm sections from FFPE samples. Sections thinner than 5 µm may be used if they can be reliably cut. Sections thicker than 10 µm are not recommended because they may not be fully digested. Also, no more than 5 sections (10 µM each) should be used for each extraction. Using too much tissue can lead to incomplete digestion and reduced yields.
Can I use tissue that is not paraffin-embedded?
Yes, tissue can be used which is not embedded in paraffin. In this case, we recommend mechanically grinding an amount of tissue equivalent to the recommended number of sections.
Can I use FFPE cores?
Yes, FFPE cores can be used. Because cores are not processed using a microtome, sample digestion tends to be more difficult and mechanical homogenization (e.g. using steel beads) is recommended if incomplete digestion is observed.
Which deparaffinization method do you recommend?
The RNAstorm™ kit includes a recommended Deparaffinization Reagent. Unlike other common methods (e.g. xylenes), the Deparaffinization Reagent is efficient, non-toxic and does not require the use of a fume hood. In our testing, the included reagent is at least as effective as xylenes at removing paraffin and allowing purification of high quality nucleic acids.
What is the best way to quantitate RNA obtained from FFPE samples?
FFPE-derived RNA is much more challenging to quantitate accurately than RNA obtained from fresh samples. It is not enough to know the absolute amount of RNA that is present, but also whether the RNA will work in downstream applications, which depends on the following factors:
- Fragment size distribution: a 5 µg sample (as measured by Qubit) can be useless for RNA-Seq if it consists of fragments < 200 nt.
- Chemical modification: for RNA obtained from formalin-fixed samples, various chemical adducts and crosslinks, including base modifications, base-base crosslinks, and base-protein crosslinks can make nucleic acid molecules inaccessible to enzymes and therefore inactive in downstream applications.
- Contamination: cellular debris, proteins, salts, and detergents used during purification can bias downstream assays. For example, UV/Vis-based methods such as Nanodrop are particularly susceptible to contaminants which absorb in the 200-280 nm range.
- Fluorescence-based methods such as Qubit are liable to significant error. When working with low concentrations of DNA or RNA, dye-based detection may not be linear. One must also be mindful of contamination by genomic DNA in an RNA sample, because the dyes used for fluorescence quantitation are not entirely specific for FFPE-derived DNA or RNA.
- Quantitative PCR is the preferred method for quantitation of heavily damaged and modified nucleic acids.
Should RIN numbers be used to determine quality of FFPE-derived RNA?
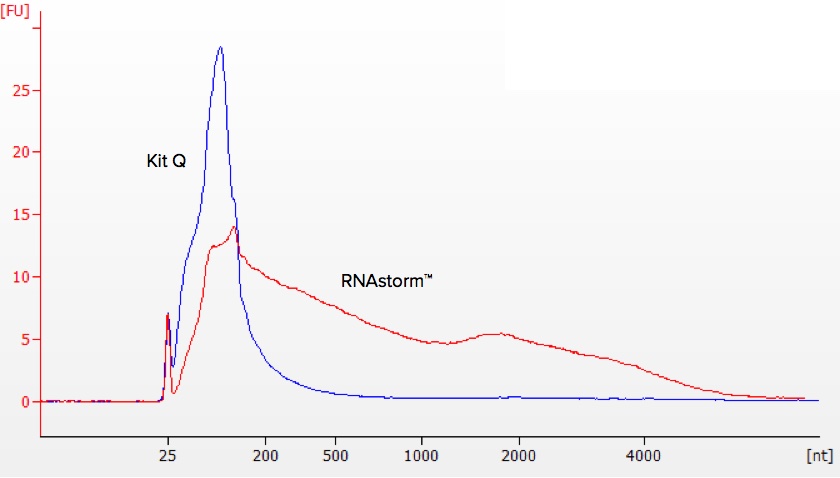
Although the RIN number can provide general information about the extent of sample fragmentation, it is not sensitive or predictable enough to be a useful indicator of downstream performance, especially for RNA-Seq. Very often, RIN numbers for FFPE-derived RNA will be between 2 and 3. Some of these samples will be useful for RNA-Seq, and others won’t - the RIN will not tell you, however.
A slightly better predictor of performance in RNA-Seq using Illumina sequencing is the DV200, which represents the percentage of RNA fragments longer than 200 nucleotides. The DV200 is also calculated based on Bioanalyzer data, but suffers from the same drawbacks as all Bioanalyzer-based methods, specifically high variability.
- Avoid methods based on organic solvents (Trizol)
- Avoid harsh chaotropic salts (i.e. guanidinium)
- Avoid detergents which impact downstream quantitation by UV and/or Qubit (e.g. Triton X-100)
- Do not rely on RIN to quantitate integrity of an FFPE-derived sample. See why. Use DV200 instead.
- Use a kit or method that removes chemical modifications from formalin. Do not raise the temperature to 80˚C or above. Even short times at this temperature will significantly lower integrity.
- Be wary of Qubit and Nanodrop concentrations because of the possibility of contamination by organic molecules or DNA.
- Use qPCR to quantitate your RNA, and always look carefully at melt curves to determine whether nonspecific amplification may have occurred.